Dr Cecile Crosnier
MRC Career Development Award Fellow
Profile
My active research interests are in the area of global health and particularly parasitic infectious diseases that have a devastating effect on the livelihood of people living in the most disadvantaged parts of the world. My group uses biochemical approaches and recombinant protein technologies to investigate host:parasite interactions and identify new vaccine candidates.
I graduated in Human Genetics and obtained my PhD at the Paris 7 – Denis Diderot University (France), working on the role of the JAGGED1 surface receptor in Alagille syndrome, a pathology of the Notch signalling pathway. As a Marie Curie postdoctoral fellow, I then moved to Cancer Research UK, London to study the role of Notch signalling in intestinal cell differentiation before joining the Wellcome Sanger Institute as a senior staff scientist where I expanded my work on cell surface proteins to study host:pathogen interactions. I used high-throughput approaches to select and recombinantly express in mammalian cells membrane-tethered and secreted proteins, which represent the cell-surface repertoire of parasites and their host cells. My work initially focussed on Plasmodium falciparum, responsible for the most severe form of malaria, and its interaction with human erythrocytes, which led to the identification of the Rh5:Basigin ligand:receptor pair that is essential for parasite entry into the red blood cell. I also developed a large library of recombinant cell surface and secreted proteins from Schistosoma mansoni, responsible for intestinal schistosomiasis, which I used in serological and vaccine studies.
I moved to the University of York in 2021 and was awarded an MRC Career Development Award to continue my work on Schistosoma mansoni and, in particular, to investigate how the parasite can manipulate its host’s immune response. The aim of this work is to shed light on new vaccine candidates against schistosomiasis and provide additional tools in the control of allergic and auto-immune responses.
Research
Schistosomiasis remains one of the most intractable, underfunded and highly-morbid parasitic diseases afflicting mankind. Its aetiological agents are flatworms of the genus Schistosoma, of which Schistosoma mansoni is the most widely distributed geographically. Despite its high and widespread incidence, there is currently no licenced vaccine and most of the control strategies in endemic populations rely on the mass administration of a single drug, praziquantel, raising the prospect of parasite resistance. Unlike many other infectious diseases, very few vaccine candidates have entered clinical trials. Parasite burdens in schistosomiasis-endemic populations show an uneven distribution across age groups as they rapidly increase during childhood and adolescence before gradually declining in adults. As a result, it has been suggested that individuals repeatedly exposed to schistosomes can develop some protective immunity to re-infection. In some cases, therapeutic intervention through praziquantel treatment can lead to drug-induced resistance (DIR) to re-infection. Protection to re-infection through antibody-mediated mechanisms has been demonstrated by serum transfer experiments in animal models but the identity of the parasite antigens targeted by the host immune system remains unknown. One of the major goals of my research is to identify the parasite antigens that are the targets of protective immunity to develop them as vaccines.
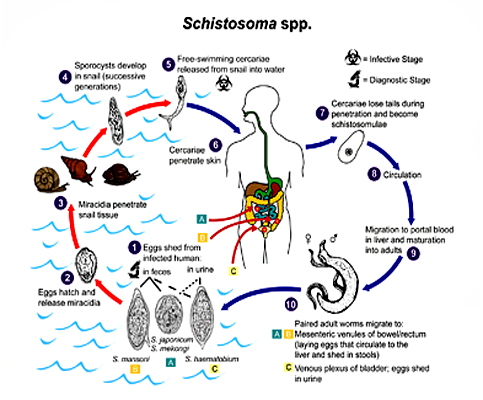
Image source: Centers for Disease Control and Prevention (CDC).
The Schistosoma life cycle
Schistosomiasis is contracted after contact with infected water containing cercariae, the free-swimming form of the parasite. After penetration through the human skin, Schistosoma larvae enter the circulatory system where they develop and mate.S. mansoni adult pairs reside in the mesenteric venules of the bowel and rectum. Trapping of parasite eggs in host tissues is responsible for the main pathological symptoms of schistosomiasis including hepato- and spleno-megaly, and liver fibrosis.
Cell surface and secreted proteins are the main mediators of intercellular communications within and between organisms and consequently, are the primary actors of host:parasite interactions and the primary targets of host antibodies. Functional studies on this class of proteins critically rely on the ability to produce correctly folded recombinant proteins that are as close as possible in structure to their native form. The formation of disulphide bonds, in particular, is essential for their correct folding and is promoted by using specific heterologous expression systems such as mammalian or insect cells as opposed to bacterial, yeast or cell-free systems. Using mammalian cells, we have successfully compiled a library of over 100 biochemically-active cell-surface and secreted proteins from Schistosoma mansoni, and demonstrated they contain heat-labile conformational epitopes. We have used these proteins in vaccine studies, and to identify early serological markers of exposure to the parasite in human controlled infections.
The aim of our research group is to use this large resource of recombinant parasite proteins in serological, biochemical and cellular assays to identify the targets of protective immunity as well as the proteins involved in host immunoregulation. By using this approach, we aim to further our understanding of immune modulation, which could have broader impact in the field of allergy and auto-immune diseases, and provide novel leads in the development of a much-needed schistosomiasis vaccine.

