Publications
56) Synthesis of highly substituted 2-spiropiperidines.
S. D. Griggs, N. Thompson, D. T. Tape, M. Fabre and P. A. Clarke, Org. Biomol. Chem. 2018, 16, 6663.
Invited Article
55) Strategies for the synthesis of spiropiperidines - a review of the last ten years.
S. D. Griggs, D. T. Tape and P. A. Clarke, Org. Biomol. Chem. 2018, 16, 6620.
News Article Reported in the Daily Mail on 14th Sept 2017 and other media outlets
54) Prebiotic Synthesis of 2-Deoxy-D-Ribose from Interstellar Building Blocks Promoted by Amino Esters or Amino Nitriles.
A. M. Steer, N. Bia, D. K. Smith and P. A. Clarke, Chem. Comm. 2017, 53, 10362.
HIGHLIGHTED in Synfacts 2017, 13, 0909.
53) A two-step synthesis of 2-spiropiperidines.
S. D. Griggs, N. Thompson, D. T. Tape, M. Fabre and P. A. Clarke, Chem. Eur. J. 2017, 23, 9262.
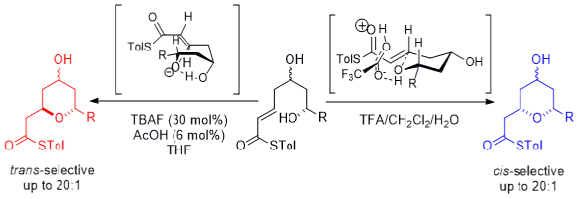
52) The stereodivergent formation of 2,6-cis and 2,6-trans tetrahydropyrans: experimental and computational investigation of a thioester oxy-Michael cyclization.
K. Ermanis, Y.T. Hsiao, U. Kaya, A. Jeuken and P. A. Clarke, Chem. Sci. 2017, 8, 482.
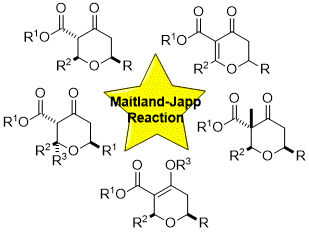
51) Synthesis of 2,6-trans and 3,3,6-trisubstituted tetrahydropyran-4-ones from Maitland-Japp derived 2H-dihydropyran-4-ones: a total synthsis of diospongin B.
P. A. Clarke, N. M. Nasir, P. B. Sellars, A. M. Peter, C. A. Lawson and J. L. Burroughs, Org. Biomol. Chem. 2016, 14, 6840.
50) Mechanistic interrogation of the asymmetric lithiation-trapping of N-thiopivaloyl azetidine and pyrrolidine.
P. J. Rayner, J. C. Smith, C. Denneval, P. O'Brien, P. A. Clarke and R. A. J. Horan, Chem. Commum. 2016, 52, 1354.
49) A Maitland-Japp inspired synthesis of dihydropyran-4-ones and their stereoselective conversion to functionalised tetrahydropyran-4-ones.
P. A. Clarke, P. B. Sellars and N. M. Nasir, Org. Biomol. Chem. 2015,13, 4743.
48) Strategies for the Construction of Tetrahydropyran Rings in the Synthesis of Natural Products.
N. M. Nasir, K. Ermanis and P. A. Clarke, Org. Biomol. Chem. 2014,12, 3323.
INVITED REVIEW of the Group's own work
47) The Development of Pot, Atom and Step Economic (PASE) Synthesis of Functionalised Tetrahydropyrans, Dihydropyrans and Piperidines.
P. A. Clarke and K. Ermanis, Curr. Org. Chem. 2013, 17, 2025.
46) Synthesis of the C20 - C32 Tetrahydropyran Core of the Phorboxazoles and the C22 Epimer via a Stereodivergent Michael Reaction.
P. A. Clarke and K. Ermanis, Org. Lett. 2012, 14, 5550.
News Article Reported in the Daily Mail on 28th Jan 2012
Top 10 Downloaded Paper in 2012 in Org. Biomol. Chem
45) Asymmetric Organocatalytic Formation of Protected and Unprotected Tetroses under Potentially Prebiotic Conditions.
L. Burroughs, P. A. Clarke, H. Forintos, J. A. R. Gilks, C. J. Hayes, M. E. Vale, W. Wade and M. Zbytniewski, Org. Biomol. Chem. 2012, 10, 1565.
44) Synthesis of the Complete Series of Mono Acetates of N-Acetyl-D-Neuraminic acid.
P. A. Clarke, N. Mistry and G. H. Thomas, Org. Biomol. Chem. 2012, 10, 529.
43) Diastereoselective synthesis of functionalised 2-methyl tetrahydropyrans.
P. A. Clarke, P. B. Sellars and N. Mistry, Tetrahedron Lett. 2011, 52, 3654.
HIGHLIGHTED in Synfacts 2011, 10, 1094.
Invited Article Tetrahedron Symposium-in-Print
42) An Asymmetric Maitland-Japp Reaction: A Highly Enantioselective Synthesis of Tetrahydropyran-4-ones.
M. Iqbal, N. Mistry and P. A. Clarke, Tetrahedron, 2011, 67, 4960.
Editorial Tetrahedron Symposium-in-Print
41) Strategies for the Synthesis of Tetrahydropyran-containing Natural Products: A Preface.
P. A. Clarke, Tetrahedron, 2011, 67, 4959.
40) Studies on Transannulation Reactions Across a Nine-Membered Ring: the Synthesis of Natural Product-like Structures.
M. Iqbal, R. J. G. Black, J. Winn, A. T. Reeder, A. J. Blake and P. A. Clarke, Org. Biomol. Chem. 2011, 9, 5062.
39) Synthesis of an Austrodorane Sesquiterpenoid Core via a Transannular Prins Cyclization.
P. A. Clarke and J. Winn, Tetrahedron Lett. 2011, 52, 1469.
38) The Asymmetric Maitland-Japp Reaction and its Application to the Construction of the C1-C19 bis-Pyran Unit of Phorboxazole B.
P. A. Clarke, S. Santos, N. Mistry, L. Burroughs and A. C. Humphries, Org. Lett. 2011, 13, 624.
37) Synthetic Studies on the phorboxazoles: a short synthesis of an epi-C23 tetrahydropyran core.
P. A. Clarke, J. M. Hargreaves, D. J. Woollaston and R. M. Rodriguez Sarmiento, Tetrahedron Lett. 2010, 51, 4731.
Hot Article 17th June 2010
36) Efficient Asymmetric Organocatalytic Formation of Erythrose and Threose under Aqueous Conditions.
L. Burroughs, M. E. Vale, J. A. R. Gilks, H. Forintos, C. J. Hayes and P. A. Clarke, Chem. Comm., 2010, 46, 4776.
35) Investigation of Transannelation Reactions across a Cyclononene Ring.
P. A. Clarke, R. J. G. Black and M. Iqbal, Synlett 2010, 543.
34) Studies on the Synthesis of the ABC-rings of (±)-Hexacyclinic acid.
P. A. Clarke, A. P. Cridland, G. A. Rolla, M. Iqbal, N. P. Bainbriddge, A. C. Whitwood and C. Wilson, J. Org. Chem. 2009, 74, 7812.
Invited Article
33) Transannulation Reactions in the Synthesis of Natural Products.
P. A. Clarke, A. T. Reeder and J. Winn, Synthesis 2009, 691.
Invited Article
32) Pot, Atom and Step Economic (PASE) Synthesis of Highly functionalised Piperidines: A Five-Component Condensation.
P. A. Clarke, A. V. Zaytsev and A. C. Whitwood, Synthesis 2008, 3530.
Highlighted in Synfacts 2008, 11, 1026
31) One-Pot synthesis of Functionalized Piperid-4-ones. A Four-Component Condensation.
P. A. Clarke, A. V. Zaytsev, T. W. Morgan, A. C. Whitwood and C. Wilson, Org. Lett. 2008, 10, 2877.
30) An Improved Synthesis of (2E,4Z)-6-(benzyloxy)-4-bromo-2,4-dien-1-ol.
P. A. Clarke, G. A. Rolla, A. P. Cridland and A. A. Gill, Tetrahedron 2007, 63, 9124.
Highlighted in Synfacts 2007, 10, 1031
29) Pot, Atom and Step Economic (PASE) Synthesis of Highly functionalised Piperidines: A Five-Component Condensation.
P. A. Clarke, A. V. Zaytzev and A. C. Whitwood, Tetrahedron Lett. 2007, 48, 5209.
28) Combining Pot, Atom and Step Economy (PASE) in Organic Synthesis: Synthesis of Tetrahydropyrans.
P. A. Clarke, S. Santos and W. H. C. Martin, Green Chem. 2007, 9, 438.
Invited Article. Top Ten Downloaded Article from EurJOC in 2006
27) Strategies for the formation of Tetrahydropyran Rings in the Synthesis of Natural Products.
P. A. Clarke and S. Santos, Euro. J. Org. Chem. 2006, 2045.
26) Enantioselective synthesis of the bicyclo[4.3.0]nonane ring system of the pinguisane-type sesquiterpenoids via a Brønsted acid promoted transannular enol alkylation.
P. A. Clarke, R. J. G. Black and A. J. Blake, Tetrahedron Lett. 2006, 47, 1453.
25) A Racemic Synthesis of an AB-Ring System of Hexacyclinic acid.
P. A. Clarke and A. P. Cridland, Org. Lett., 2005, 7, 4221.
Top Ten Downloaded Article from OBC in September 2005
24) The One-Pot, Multi-Component Construction of Highly Functionalised Tetrahydropyran-4-ones using the Maitland-Japp Reaction.
P. A. Clarke, W. H. C. Martin, J. M. Hargreaves, C. Wilson and A. J. Blake, Org. Biomol. Chem., 2005, 3, 3551.
23) Exploiting the Maitland-Japp Reaction: A Synthesis of (±)-centrolobine.
P. A. Clarke and W. H. C. Martin, Tetrahedron, 2005, 61, 5433.
22) Synthesis of the B-Ring of FR182877. Investigation of the Reactions of 6-fumaryl 1,3,8-nonatrienes.
P. A. Clarke, R. L. Davie and S. Peace, Tetrahedron, 2005, 61, 2335.
21) Revisiting the Maitland-Japp reaction. Concise construction of highly functionalised tetrahydropyran-4-ones.
P. A. Clarke, W. H. C. Martin, J. M. Hargreaves, C. Wilson and A. J. Blake, Chem. Comm., 2005, 1061.
20) Synthetic Studies on the DEF-rings of FR182877 and Hexacyclinic acid.
P. A. Clarke, M. Grist, M. Ebden, C. Wilson and A. J. Blake, Tetrahedron, 2005, 61, 353.
19) An expedient synthesis of (±)-centrolobine
P. A. Clarke and W. H. C. Martin,Tetrahedron Lett., 2004, 45, 9061.
18) Total Synthesis Highlights.
P. A. Clarke and A. P. Cridland, Annu. Rep. Prog. Chem., Sect. B, 2004, 100, 91.
17) Process for the Selective Derivatization of Taxanes
P. A. Clarke, R. A. Holton and Z. Zhang, US Patent: US 6,706,896 B1, March 16, 2004.
16) An iodocyclisation/elimination approach to a DEF-ring core of FR182877.
P. A. Clarke, M. Grist and M. Ebden, Tetrahedron Lett., 2004, 45, 927.
15) Mechanistic Insight into the Lanthanide(III) salt Catalysed Monoacylation of Symmetrical Diols from Structural Models.
P. A. Clarke, P. L. Arnold, M. A. Smith, L. S. Natrajan, C. Wilson and C. Chan, Chem. Comm., 2003, 2588.
14) Synthetic Methods Part (v): Protecting Groups.
P. A. Clarke and W. H. C. Martin, Annu. Rep. Prog. Chem., Sect. B, 2003, 99, 84.
13) Synthesis of a Model DEF-Ring Core of Hexacyclinic acid.
P. A. Clarke, M. Grist, M. Ebden and C. Wilson, Chem. Comm., 2003, 1560.
12) Diastereoselective Synthesis of Highly Substituted Tetrahydropyran-4-ones.
P. A. Clarke and W. H. C. Martin, Org. Lett, 2002, 4, 4527.
11) A One Step Procedure for the Mono-Acylation of Symmetrical 1,2-Diols.
P. A. Clarke, N. E. Kayaleh, M. A. Smith, J. R. Baker, S. J. Bird and C. Chan, J. Org. Chem , 2002, 67, 5226.
10) Selective mono-acylation of meso- and C2-symmetric 1,3- and 1,4- diols.
P. A. Clarke, Tetrahedron Lett., 2002, 43, 4761.
9) On the Diels-Alder reactions and the Lewis acid induced rearrangements of 6-fumaryl 1, 3, 8- nonatrienes.
P. A. Clarke, R. L. Davie and S. Peace, Tetrahedron Lett., 2002, 43, 2753.
8) Process for the Selective Derivatisation of Taxanes.
P. A. Clarke, R. A. Holton and Z. Zhang, US Patent: US 6,191,287 B1, Feb 20, 2001
7) Direct One Step Mono-Functionalisation of Symmetrical 1,2-Diols.
P. A. Clarke, R. A. Holton and N. E. Kayaleh, Tetrahedron Lett., 2000, 41, 2687.
6) The Carbenoid Approach to Peptide Synthesis.
R. T. Buck, P. A. Clarke, D. M. Coe, M. J. Drysdale, L. Ferris, D. Haigh, C. J. Moody, N. D. Pearson and E. Swann, Chem. Eur. J., 2000, 6, 2160.
5) Process for the Selective Derivatisation of Taxanes.
P. A. Clarke, R. A. Holton and Z. Zhang, World Wide Patent Applications 1998, 1999, 2000: based on US Patent: US 6,191,287 B1.
4) Selective Protection of the C(7) and C(10) Hydroxyl Groups in 10-Deacetyl Baccatin III.
R. A. Holton, Z. Zhang, P. A. Clarke, H. Nadizadeh and D. J. Procter, Tetrahedron Lett., 1998, 39, 2883.
3) Ketone Directed Peracid Epoxidations of Cyclic Alkenes.
A. Armstrong, P. A. Barsanti, P. A. Clarke and A. Wood, J. Chem. Soc., Perkin Trans. 1, 1996, 1373.
2) Evidence that a Dioxirane is not responsible for Alkene Epoxidation in a Ketone - Oxone® System.
A. Armstrong, P. A. Clarke and A. Wood, Chem. Comm., 1996, 849.
1) Ketone Directed Peracid Epoxidations.
A. Armstrong, P. A. Barsanti, P. A. Clarke and A. Wood, Tetrahedron Lett., 1994, 35, 6155.