Professor Paul Walton
Email: paul.walton@york.ac.uk
Research: Bioinorganic chemistry
Lytic Polysaccharide Monooxygenases (LPMOs) and the histidine brace
LPMOs, along with other enzymes, have a copper-containing active site in an N-terminal histidine (Proc. Nat. Acad. Sci., 2011) coordinates to the copper. The active site was discovered in 2011 and is known as the histidine brace. LPMOs catalyse the oxidation of recalcitrant polysaccharides such as cellulose. Our current research interests involve understanding the catalytic mechanisms of these enzymes using a combination of structure, spectroscopy and theory, J. Am. Chem. Soc. 2023, and also revealing the wider role of LPMOs in biology, Science 2021, 774-779.
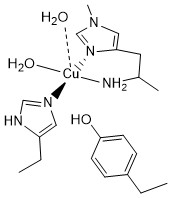
LPMOs shown to have protective charge transfer pathway
Working with colleagues at the University of Manchester, we were able to determine the role of the active site tyrosine found in many LPMOs, J. Am. Chem. Soc. 2023. Using a combination of stopped flow spectroscopy, freeze-quench, EPR spectroscopy and HERF-D X-ray absorption spectroscopy, we demonstrated that the tryosine is oxidised during hydrogen-atom transfer to the histidine brace active site, which itself had become damaged during uncoupled turnover of the enzyme. As such, the tyrosine provides a means of protecting the active site of LPMOs from inadvertent oxidation. This work is related to earlier work we did on the formation of tyrosine radicals in LPMOs (J. Am. Chem. Soc. 2019).
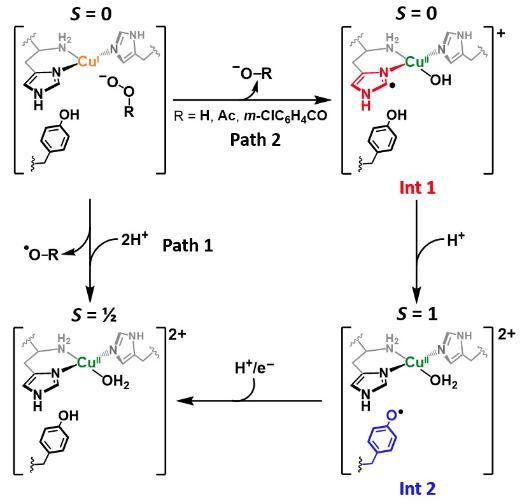
Figure. Reaction pathway for formation of protective tyrosyl radical in LPMOs
O2-substrate coupling mechanism in chitin-active LPMOs
A combined NMR/EPR study unravels the mechanism by which the activation of oxygen is coupled to the binding of substrates in LPMOs. We use an analysis of EPR hyperfine constants to show that the binding of chitin by LPMOs is accompanied by a shift in the coordination geometry at the copper ion. This work is published in PNAS.
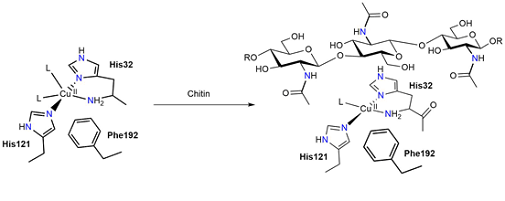
Figure: Changes in the active site structure of chitin-active LPMOs upon binding of substrate.
Recent publications
- Secreted pectin monooxygenases drive plant infection by pathogenic oomycetes, F Sabbadin, S Urresti, B Henrissat, A O Avrova, L R J Welsh, P J Lindley, M Csukai, J N Squires, P H Walton, G J Davies, N C Bruce, S C Whisson, S J McQueen-Mason, Science, 2021, 373(6556), 774-779.
- Mapping the Initial Stages of a Protective Pathway that Enhances Catalytic Turnover by a Lytic Polysaccharide Monooxygenase, , J. Am. Chem. Soc. 2023, 145(37), 20672-20682 (open access article).
- Mechanistic basis of substrate-O2 coupling within a chitin-active lytic polysaccharide monooxygenase: an integrated NMR/EPR study, G Courtade, L Ciano, A Paradisi, P J Lindley, Z Forsberg, M Sorlie, R Wimmer, G J Davies, V G H Eijsink, P H Walton, F L Aachmann, Proc. Nat. Acad. Sci. 2020, 117(32), 19178-19189 (open access article).
- A fungal family of lytic polysaccharide monooxygenase-like copper proteins, A Labourel, K Frandsen, F Zhang, N Brouilly, S Grisel, M Haon, L Ciano, D Ropartz, M Fanuel, F Martin, D Navarro, M-N Rosso, T Tandrup, B Bissaro, K Johansen, A Zerva, P H Walton, B Henrissat, L Leggio, J-G Berrin, Nature Chem. Biol. 2020, 16, 345-350.
- Formation of a copper(II)-tyrosyl complex at the active site of lytic polysaccharide monooxygenases following oxidation by H2O2, A Paradisi, E M Johnston, M Tovborg, C R Nicoll, L Ciano, A Dowle, J McMaster, Y Hancock, G J Davies, P H Walton, J. Am. Chem. Soc., 2019, 141, 18585-18599 (open access article).
- Bracing copper for the oxidation of C-H bonds, L Ciano, G J Davies, W B Tolman, P H Walton Nature Catalysis, 2018, 1(8), 571-577.
Biography
Paul Walton obtained his PhD in 1990 (University of Nottingham, UK), followed by two years as a NATO/SERC postdoctoral fellow at the University of California, Berkeley, USA. He joined the Department of Chemistry at York as a faculty member in 1993. Between 2004 and 2010 he was chair of department. His main research area is bioinorganic chemistry, in which he has made contributions to the understanding of copper oxidases, including the discovery of the histidine brace.
He is the recipient of multiple national* and international** awards, including:
Teaching: RSC's Higher Education Teaching Award,** Vice-Chancellor's Teaching Award.
Research: Gertrude Cropper Award, RSC's Joseph Chatt Award,** IChemE's Global Energy Award,** RSC's Rita and John Cornforth Award,** University of Chalmers Jubilee professor 2020.**
Equality: Royal Society's inaugural Athena Prize* (runner-up). WISE man of the year shortlist.*
He has also been Editor of Dalton Transactions (2004-2008), chair of Heads of Chemistry UK, chair of the Royal Society of Chemistry's Diversity Committee, was named as a 'Person of Influence' by the University of Toronto's Women in Chemistry Group and is one of the RSC's 175 Faces of Chemistry. Paul is an internationally-known advocate of equality in sciences and lectures widely on the subject.

