Dr Charlotte Willans
Reader in Synthetic Inorganic Chemistry
Overview
Dr Charlotte Willans is a Reader in Synthetic Inorganic Chemistry. Research within the group involves the exploration of organometallic complexes in both catalytic and biomedicinal applications.
Mechanistic understanding of processes using both precious- and base-metal systems aids in the development of ligand architectures for overall enhanced efficacy.
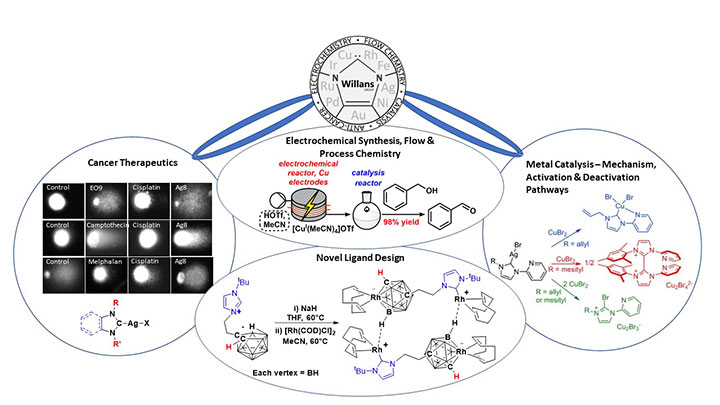
New approaches for the synthesis of metal species using electrochemistry enables more sustainable routes towards these complexes, with translation of the methodology into flow technology enabling a high-throughput approach to their development.
Biography
Biography
Dr Charlotte Willans obtained her MChem degree in 2002 from the University of York, conducting a research project at DSM (Netherlands) with Professors Johannes de Vries and André de Vries on palladium-catalysed cross-coupling reactions.
She completed her PhD in 2006 at the University of York working with Professor Francesca Kerton and Professor Jason Lynam on lanthanide catalysis and organophosphorus cages.
She completed a postdoctoral position with Professor Jonathan Steed in Durham (2006-2009), working on MOFs and metal-N- heterocyclic carbenes, in collaboration with Professor Len Barbour in Stellenbosch, South Africa.
Charlotte was awarded a Royal Society Dorothy Hodgkin Fellowship which she took to Leeds in 2009. She was awarded a Leeds University Research Fellowship in 2013 and was promoted to Associate Professor in 2018. In 2023 Charlotte moved to the University of York where she is a Reader in Synthetic Inorganic Chemistry.
Research
Research
Electrosynthesis and Flow Technology
Several routes are known for the preparation of NHC and other organometallic complexes. These generally require the use of strong bases, strict inert conditions and result in undesirable side-products such as metal salts. In addition, problems are often encountered when the ligand precursor contains several acidic protons. We have developed an electrochemical synthetic route to metal complexes which is fast and high yielding, with the only side-product being hydrogen (Chem. Commun. 2012, 48, 4887). We have found that the range of ligands that can be used in this method is diverse (Organometallics 2013, 32, 570; ChemistryOpen 2016, 5, 351), and we expect it to become a common easy access route to organometallic and coordination compounds.
We have designed, built and optimised electrochemical flow reactors for the synthesis of metal-NHCs and other organometallic and coordination complexes (Chem. Commun. 2015, 51, 1282; Inorg. Chem. 2021, 60, 6976). Our flow technology makes the route highly efficient and easily scalable, and provides a safer and more sustainable methodology to metal species.
As part of an EPSRC-funded project, we have integrated our electrochemical technology into a continuous flow platform for high-throughput electrochemical synthesis, screening and optimisation of metal catalysts (Catal. Sci. Tech. 2022, 12, 4266). This will significantly speed up the rate at which catalysts are discovered, optimised and implemented in industrially relevant reactions. As part of this we have gained better understanding of the effects of flow reactor channel shape (React. Chem. Eng. 2022, 7, 264) and alternating polarity (React. Chem. Eng. 2021, 6, 147) in electrochemical reactors. The work forms part of ongoing and very fruitful collaborations with Professor Nik Kapur, Professor Richard Bourne and Dr Bao Nguyen (University of Leeds).
See our Tutorial Review in Green Chemistry for an accessible introduction into electrosynthesis for synthetic chemists: ‘Making electrochemistry easily accessible to the synthetic chemist’.
Base Metal Catalysis
Many catalytic reactions used in the pharmaceutical industry rely on costly and potentially toxic precious metals. In a drive to lower the cost of these processes and improve sustainability, research efforts are focused on using base-metals such as copper and iron. These reactions often require high loadings due to side reactions and catalyst deactivation. We are using the unique properties of NHC ligands to stabilise base metal-NHCs in different oxidation states, with the aim of stabilising catalytically active intermediates or redirecting catalysis via an alternative and desired pathway.
We are keen to understand the non-innocent behaviour of NHCs at metals, with a particular focus on identifying deactivation of NHCs at copper and iron. We collaborate with Professor Alireza Ariafard (Tasmania) to support our experimental work with computational calculations (Chem. Commun. 2016, 52, 5057; Organometallics 2015, 34, 3497; Chem. Eur. J. 2014, 20, 12729).
We have reviewed non-spectator NHCs in Organometallic Chemistry: ‘N-Heterocyclic carbenes; partakers not just spectators’, in addition to a more recent book chapter in Advances in Organometallic Chemistry: ‘Reactivities of N-heterocyclic carbenes at metal centres’.
We are working with Dr Bao Nguyen (University of Leeds) to identify deactivation pathways to enable us to address key problems faced in base metal-catalysed processes (Chem. Sci. 2017, 8, 7203; J. Am. Chem. Soc. 2015, 137, 4151).
Precious Metal Catalysis
Whilst significant research effort is focussed on developing base metal catalysts, precious metals such as palladium, rhodium and iridium are still the go-to elements for industry processes and will be business critical for many years to come. To enable valuable resources to be used in a more efficient and sustainable way, our aim is to understand mechanisms and deactivation pathways within precious metal catalysed reactions and use this knowledge to design more efficient catalysts and processes.
For example, catalyst speciation in palladium-catalysed cross-coupling reactions affects reaction selectivity, with the possibility to control speciation using ligand design thus control activity and selectivity (Dalton Trans. 2019, 48, 14687; J. Am. Chem. Soc. 2021, 143, 9682). This has led to a research project in collaboration with Professor Ian Fairlamb (York) to assess the effects of NHC ligands on palladium speciation during catalysis.
Iridium
Bioorganometallic Chemistry
In collaboration with Professor Roger Philips, Professor of Cancer Pharmacology at the University of Huddersfield, we were one of the first groups to assess the cytotoxic behaviour of silver-N-heterocyclic carbene (NHC) complexes (Dalton Trans. 2021, 41, 3720). We have investigated structure-activity relationships, selectivity and developed new ligand architectures to reduce toxicity, such as those derived from the natural product caffeine (Dalton Trans 2015, 44, 7563). Through the award of a Yorkshire Cancer Research Grant, we explored the mechanism of action of our complexes on cancer cells, uncovering multiple pathways of importance in cancer biology and lower activity in non-cancer cells (Cancer Lett. 2017, 403, 98).
In collaboration with Dr Paul Thornton, a polymer chemist in Leeds, we have encapsulated our silver-NHC complexes in polymer micelles, with the aim of protecting, delivering, and releasing the drugs at the site of action (RSC Advances 2018, 8, 10474). We have also collaborated with Professor Andrew Nelson in Leeds to assess our complexes on an artificial bio-membrane to compare membrane disruption with cytotoxicity (Organometallics 2020, 39, 1318).
As part of a comprehensive study into the antibacterial effects of a range of metal complexes, one of our silver-NHC complexes exhibited the best broad-spectrum activity from 30 different metal complexes tested. Different silver complexes showed distinct profiles, indicating that the ligand plays a key role in biological behaviour (Chem. Sci. 2020, 11, 2627).
Novel Ligand Design
In collaboration with Dr Mark Fox (Durham), we were the first to report NHCs fused with a carborane, in which the NHCs stabilised a very unusual Lewis base boron atom (Chem. Eur. J. 2010, 16, 10644). This led to further collaborative work that developed new ligand architectures combining NHCs with carboranes (Chem. Commun. 2016, 52, 6443) that were applied in both cancer therapeutics (Organometallics 2019, 38, 2530) and catalysis (Dalton Trans. 2016, 45, 15818).