C-F Bond Activation and Metal Fluoride Complexes
Routes to new fluoro-organics
If transition metals can insert into the C–H bonds of methane and benzene, why not insert into the even stronger C–F bonds of fluorocarbons? This argument started our research into C–F bond activation. By now we have found C–F bond activation reactions with elements from rhenium to nickel.1 Some mechanisms parallel those of C–H activation, others do not. Those reactions that result in C–F oxidative addition offer one of the few methods of synthesising metal fluoride complexes. The reactions of fluoropyridines and other hetero-aromatics have provided routes to new fluoro-organic compounds formed by reaction of metal aryl fluoride complexes, for instance with a carbanion. Another spin-off has been the discovery of complexes of bifluoride (FHF) formed by release of HF in C–F activation reactions. The HF is trapped by forming a hydrogen bond at a metal fluoride. The bifluoride complexes can be characterised in solution and the crystal and are highly dynamic in solution.2
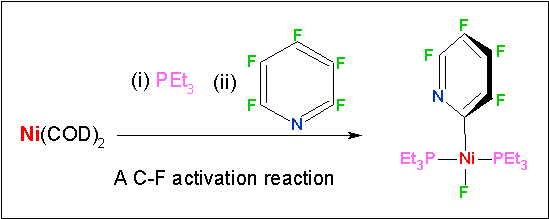
In recent research, we have shown that zero oxidation state complexes of nickel, palladium and platinum all react in different ways with pentafluoropyridine.3 The nickel reaction is shown in the box below, palladium reacts much more slowly and reacts at the 4-position of the heterocycle. Platinum reacts totally differently undergoing a rearrangement that cleaves a phosphorus carbon bond forms a metal alkyl and a fluorophosphine complex (see structure below). We have also used theoretical methods to compare the energetics and kinetic barriers for C–F vs C–H oxidative addition at nickel and platinum.4 The calculations reveal why nickel is the element of choice for C–F bond activation, while platinum is the best for C-H bond activation. Most recently, we reacted a rhodium silyl complex first with a fluorinated heteroaromatic and then with a diborane. The net result is borylation of a C–F bond.5 In 2006, we reviewed and classified C-F bond activation reactions (with Braun).6
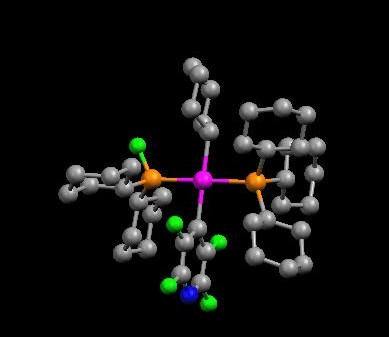
Structure of product of reaction of Pt(PCy3)2 with pentafluoropyridine
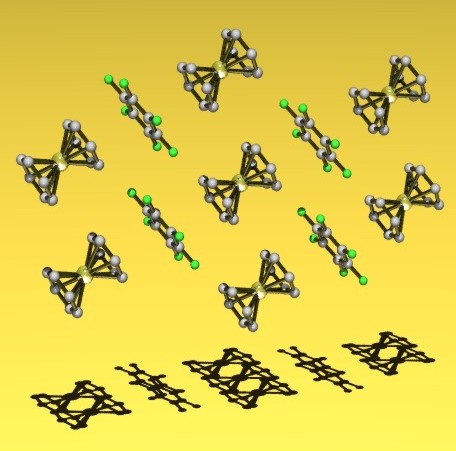
A stacking charge-transfer complex Cr(C6H6)2.C6F6
Selected Publications
- Routes to fluorinated organic derivatives by nickel-mediated C-F activation of heterocycles. Chem Commun, 2002, 2749
- Hydrogen bonding in transition metal complexes: platinum hydride bifluoride complexes. J Am Chem Soc, 2000, 122, 8685
- Contrasting reactivity of fluoropyridines at Pd and Pt Organometallics, 2004 23, 6140
- A comparison of C-F and C-H bond activation by zerovalent Ni and Pt, J Am Chem Soc, 2004, 126, 5268
- Sequential C–F borylation of fluoropyridines Chem Commun, 2007, 3664-3667.
- Transition-metal mediated C-F bond activation, Comprehensive Organometallic Chemistry III, Chapter 1.26, Elsevier 2006.
