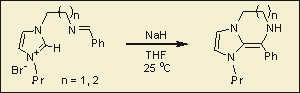Richard Douthwaite Group
Research
Our research is currently focused on the synthesis, understanding and application of materials and metal complexes for catalysis using renewable energy (mainly solar energy and renewable electricity derived from solar and wind).
Photocatalysis and Solar Energy Conversion
Photocatalysts are a class of material that mediate chemical reactions using photons as a source of energy. There are many potential uses including the degradation of chemicals detrimental to the environment, clean chemical synthesis, and the conversion of solar into chemical energy (e.g. water to dihydrogen and dioxygen).
In principle, the concept is simple: to generate reducing (electrons) and oxidizing (holes) from excitation across a bandgap (Fig. 1), which can subsequently be used for redox chemistry. However, in practice there are tremendous challenges to address across efficiency, stability, cost, and practical implementation. It is unlikely a single compound will be able to perform all the required steps efficiently (absorption across the visible spectrum, electron/hole migration to the surface, and catalysis of the chosen reaction, whilst remaining stable for a long time). Therefore 'engineered' composites are the more likely solution where more than one material can be used to perform one or two of the underpinning functions.
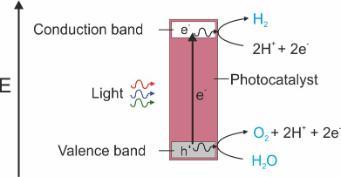
Figure 1
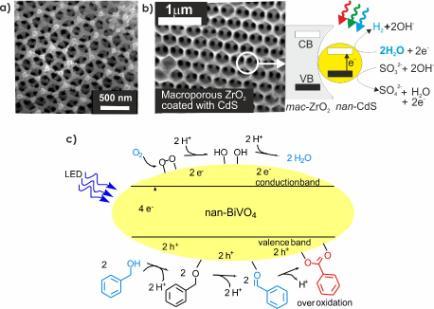
Examples from our research include macroporous TaON (Fig. 2a) and photocatalytic nanoparticles embedded in macroporous hosts (Fig. 2b) both for hydrogen production from water. Macroporous materials have interesting optical properties that can be exploited to increase the efficiency of a photocatalytic reaction. We have also studied metal oxides such as BiVO4 for selective oxidation of alcohols to aldehydes using oxygen as the chemical oxidant (Fig. 2c) avoiding the need for stoichiometric oxidants such as peroxides, manganate or chromate which require energy for synthesis or generate toxic waste.
Figure 2
Photocatalytic concepts are also relevant to photoelectrochemistry where an electrical field (bias) can be used to increase electron-hole separation and the rate of redox catalysis. This technology also supports easier product separation. We use photoelectrochemistry to study photocatalytic materials and as the basis for simple photoelectrochemical devices. Examples include 3-dimensional transparent conducting oxides such as aluminium zinc oxide spheres (Fig. 3a) which can be coated with photoactive materials for hydrogen production, and porous BiVO4 for C-C bond formation such as the Kolbe reaction (Fig. 3b). These 3-D structures allow more light to be absorbed for a given surface area, and support greater electrode-electrolyte interfacial contact compared to planar electrodes.
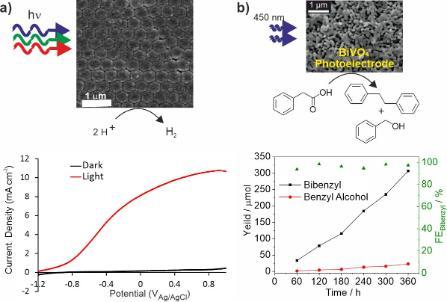
Figure 3.
Catalysis using metal complexes
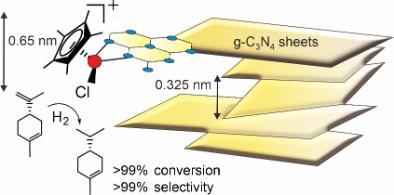
Metal complex catalysis has recently focused on synthesis and electrocatalysis of Co complexes for electrocatalytic hydrogen production and supporting Ir hydrogenation catalysts on carbon nitride (C3N4) substrates (Fig. 4). The aim here is to study structure property relationships to guide increases in activity and selectivity. For example, the Ir-C3N4 system contains Ir complexes coordinated to the edge sites of C3N4 planes which supports regioselective hydrogenation.
Figure 4.
Older projects
Microwave-Induced Plasma Materials Synthesis
We built a testing facility and microwave plasma reactor (Figure 5) to discover new materials for photocatalytic applications. The use of microwave-induced plasmas (MIP) builds on some of our earlier work exploring the synthesis of materials using microwave methods.
Microwave heating is a well established technique for solution based synthetic chemistry and most modern molecular laboratories now contain a microwave reactor. Commonly, rapid reaction rates can be achieved because of superheating that is a result of coupling between the solvent (or reactants) and microwave radiation. Similarly in the solid state one of the components of a reaction must couple with the microwaves, generating heat via dielectric or conduction losses to drive a reaction. Solids can heat at enormous rates (100 K/s), however many of interest do not couple with microwaves at room temperature limiting the application of this technique to preparative materials chemistry.
We designed and constructed a reactor capable of initiating and sustaining microwave-induced plasmas of various gases including Ar, N2, H2/N2, NH3 and O2. The plasma can be used as a source of heat to drive bulk solid state reactions and also as a source of reactive species for gas-solid reactions for surface or bulk modification.
Figure 5
New(ish) N-donor Ligands
Nitrogen based compounds are amongst the most versatile and interesting ligands in transition metal and main group chemistry supporting a wide range of reactive species and promoting stoichiometric and catalytic reactivity. Examples include proteins and porphyrins in biological metalloenzymes, amido ligands in C-H and multiple bond activation, and imines and amines in polymerization, oxidation and Lewis acid catalysis.
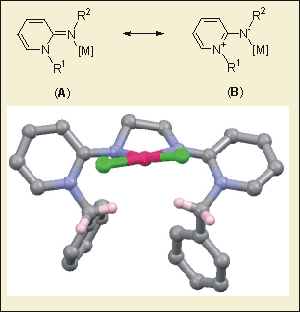
We have been interested in developing a new simple class of N-donor compounds that can be simplistically characterised as neutral amido ligands. Initial work has focused on 1H-pyridin-(2E)-ylidenes (PYEs) that are very easy to synthesize in one step. PYE type ligands appear to be strong donors and their structure renders the N-substituent (R1, Figure 6) close to the metal, which can control reactivity.
Figure 6
This project is supported by DFT studies to probe metal-ligand bonding and electron distribution across the ligand.
N-Heterocyclic Carbenes (NHCs)
Our interest in this class of compound has been to prepare new achiral and chiral compounds and investigate their application as ancillary ligands for metal-mediated catalysis. We have recently prepared a new class of enamine that results from formal addition of a NHC to an imine (Figure 7). The organic and organometallic chemistry of this very reactive molecule should be of interest for the preparation of new classes of N-heterocycles and also potentially chelating NHC-acyclic carbene complexes via insertion of a metal into the C=C double bond.
Figure 7
In addition to their growing popularity over the last 20 years for catalytic applications NHC are also of interest because of their unusual electronic properties and structure. In common with many other groups we are also interested in preparing complexes that may exhibit new stoichiometric reactions that may have future application to new catalytic processes.
Equipment
The Department of Chemistry has modern analytical facilities including NMR spectrometers (700, 600, 500, 400, 300, 270 MHz), IR, UV and ESR, fluorescence and time-resolved photoluminescence spectrometers; mass (ESI, FAB) spectrometers; powder diffraction, XRF, TGA, DSC, and BET surface area measurements. Through the York Jeol Nanocentre the group has access to an array of microscopy facilities including SEM, (S)TEM, and AFM.
In addition to standard laboratory equipment the group also has its own fibre optic UV-Vis and diffuse reflectance spectrometer, semi-prep HPLC, GC, autoclaves, carousel reactors, drybox, light sources (Xe-arc lamps and LED arrays with a monochromator for wavelength dependent measurements); a range of electrochemical workstations including Parstat 3000 and Biologic SP-200; film fabrication equipment including electrospray, spin coater, dip-coater, ozone cleaner; and contact angle measurement.