Research and publications
Working with researchers at the Universität Osnabrück, Germany, and the University of Helsinki, Finland, we have revealed how binding of the haematopoietic signaling molecule (cytokine) and thrombopoietin, to MPL on the outside of the cell brings two molecules of MPL together to transmit signals within the cell that promote cell growth.
We've also shown how mutations within MPL and its associated signaling protein, Janus kinase 2 (JAK2) can override these signals to cause uncontrolled cell division and cancer. Find out more about our research below.
We have shown that an oncogenic mutant form of Janus kinase 2, JAK2 V617F, needs to directly associate with the functional homodimeric type I cytokine receptor, MPL (the receptor for the essential cytokine thrombopoietin, THPO), in order to drive myeloproliferative neoplasms (MPNs), a group of rare and chronic blood cancers. More recently, we have used advanced live-cell imaging techniques to demonstrate that this mutant form of JAK2 drives oncogenic signalling by allowing two MPL molecules to come together in the cell membrane without the need for THPO. By identifying novel pathways that control the function of mutant JAK2, in addition to investigating how THPO signalling regulates MPN progression, we aim to design new drugs for future MPN therapies. To this end we are applying molecular and structural biology, biophysical and advanced imaging techniques in order to understand the molecular mechanisms underlying signalling through MPL in health and disease, and to then use that understanding to develop MPN therapeutics.
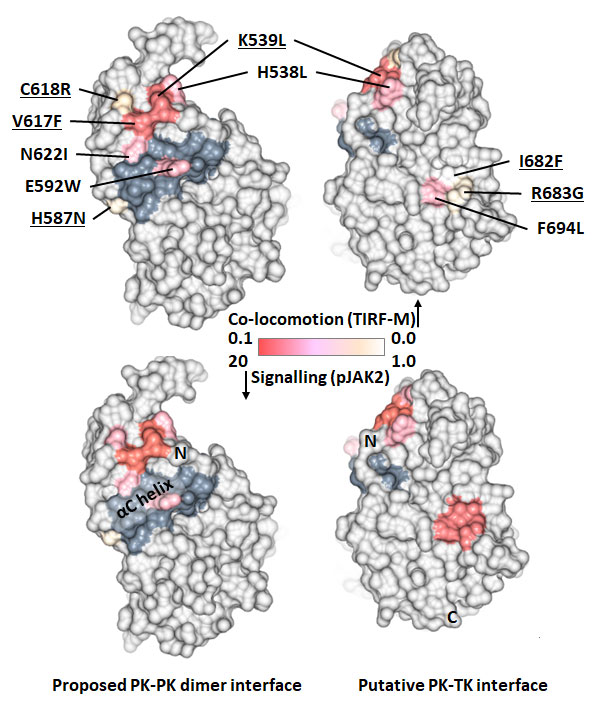
Disease-associated and artificially introduced mutations in the JAK2 pseudokinase (PK) domain mapped to the molecular surface and coloured by their effect on MPL dimerisation (measured by single-particle tracking on live cells) and cellular signalling (phosphorylated JAK2). The mutations define two separate regulatory surfaces; (i) one group of mutations (shaded darker grey) enhance signalling by stabilising an intermolecular PK domain dimer interface, whilst (ii) a second group of mutations destabilise an inhibitory intramolecular interface between the PK domain and adjacent tyrosine kinase (TK) domain. Figure created using data source 1, source 2, source 3, source 4 and CCP4MG.
Research papers
Mechanism of homodimeric cytokine receptor activation and dysregulation by oncogenic mutations
We are interested in a group of haematological malignancies called the myeloproliferative neoplasms (MPNs). We have previously demonstrated that when the oncogenic mutant form of Janus kinase 2, JAK2 V617F, which drives these diseases, is present in the endothelial cells that line the blood vessel wall, it also plays a key role in the aberrant thrombosis/ haemostasis, which is a common cause of morbidity and mortality in MPN patients.
Research papers
We look at:
- The status of the immune system in visceral leishmaniasis
- Mechanisms and functional consequences of platelet-macrophage cross-talk in type 2 immunity
We are currently using experimental in vivo disease models, flow cytometry and immunofluorescence to better understand the haematological complications that occur during chronic infection with the parasites, Leishmania donovani and Schistosoma mansoni, and to track the response of the blood and immune systems after elimination of these infections. We are interested in understanding the causes of thrombocytopenia (reduced platelet count) associated with these diseases, and in exploring the role of platelets in modulating the immune response and inflammation. We are also investigating the immune response in cases of chronic leishmaniasis where there is a secondary stressor such as another infection (for example malaria) or an immune-mediated disorder (for example immune thrombocytopenia (ITP)) to better reflect the situation experienced by many patients with the disease. The findings from these studies have the potential to influence patient treatment regimens for these increasingly prevalent neglected tropical diseases (NTDs).
Research papers
Quantitative Optical Diffraction Tomography Imaging of Mouse Platelets
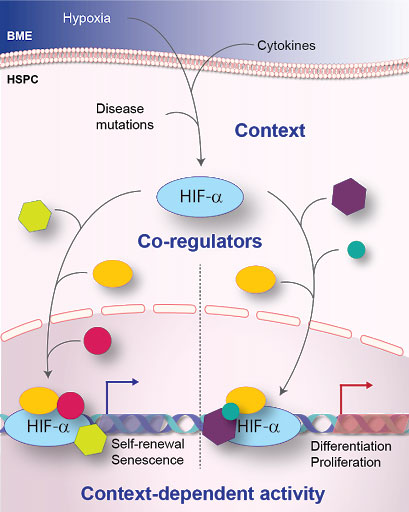
All mature blood cell lineages are derived from a pool of multi-potential parental cells called haematopoietic stem cells (HSCs). HSCs have the ability to differentiate into committed daughter cells (called progenitors), whilst retaining the ability to self-renew and thus maintain the HSC pool. Thrombopoietin (TPO) is an indispensable cytokine for HSC self-renewal (as well as megakaryopoiesis - the production of megakaryocytes - the cells that make platelets). HSCs reside in discrete anatomical locations within the bone marrow, termed niches. There is evidence that local production of TPO from specialised cells within the HSC niche occurs, yet the exact cells responsible are largely unidentified. We aim to identify the TPO-producing cells within the HSC niche and to determine to what extent they are needed for HSC self-renewal.
Katherine Bridge’s research focuses on proteins called hypoxia-inducible factors (HIFs). As transcription factors, HIFs are responsible for changing the genes which a cell expresses, which in turn changes the cell’s behavior. The mechanism by which HIFs are told which genes to express and when, is poorly understood. Understanding this mechanism is critical: in certain contexts HIFs can drive the expression of genes which underlie a number of fatal diseases, including heart disease, stroke and cancer. In others, HIFs can be protective against these diseases. Katherine is using blood stem cells (HSCs) as a model system to discover these mechanisms. This knowledge will unlock the potential of reprogramming HIFs which are driving a disease into HIFs which are preventing the very same disease, holding vast potential for reprogramming these master regulators into forces for good.