Professor Nia Bryant
Chair of Cell Biology
Research
Control of Intracellular Membrane Traffic
A defining feature of eukaryotic cells is their compartmentalization into discrete membrane-bound cytosolic organelles. While each organelle maintains its own unique complement of macromolecules necessary for its particular function, there is a high level of communication (exchange of material) between these various intracellular compartments. This is facilitated through membrane fusion events, in many cases by vesicular transport where cargo molecules, both membrane-bound and soluble, are packaged into vesicles that bud from the donor compartment. These vesicles then dock and subsequently fuse with the appropriate target organelle, delivering their contents.
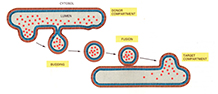
Non-disruptive transportation of molecules between these organelles and to the plasma membrane is extremely important, and each trafficking event is tightly regulated both spatially and temporally – i.e. it is imperative that each transport vesicle docks and fuses with the right target compartment at the right time. SNARE (soluble N-ethylmaleimide-sensitive fusion protein-attachment protein receptor) proteins are central to this process, facilitating fusion by formation of specific complexes between SNAREs on opposing lipid bilayers, through their highly conserved α-helical cytosolic SNARE motifs.
Regulating SNARE complex assembly provides the cell with a means to regulate membrane traffic. Understanding this process is important, as it underpins many physiological and developmental processes and is dysregulated in numerous disease states, including cancers, diabetes and neurodegenerative disorders.
As with many processes in eukaryotic cell biology, the molecular mechanisms that regulate membrane traffic are conserved through evolution from yeast to humans. We therefore use the genetically tractable model eukaryote Saccharomyces cerevisiae (Baker’s yeast) to study human diseases such as neutropenia (which we have mapped to a defect in the SNARE regulator Vps45).
|
This figure shows 3T3-L1 adipocytes expressing a version of GLUT4 harbouring an HA-epitope tag in its first exofacial loop treated with 100nM insulin |
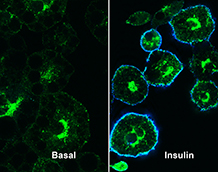 |
We are particularly interested in how the hormone insulin regulates membrane traffic in fat and muscle cells. This is an important goal, as defects in this system underlie Type-2 Diabetes, a debilitating disease whose incidence is increasing worldwide at an exponential rate. One of the major actions of insulin is to bring about changes in the membrane trafficking of a glucose transporter called GLUT4 that deliver it to the cell surface. We have discovered that this is achieved by altering the way in which SNARE complexes are formed. As this system is dysfunctional in individuals suffering from Type-2 Diabetes, this discovery has diagnostic and therapeutic potential.
Another exciting project in our lab involves using our knowledge of membrane traffic to enhance the secretory capacity of cells that are used to produce monoclonal antibodies for therapeutic purposes. This has the potential to greatly reduce the cost of these medicines and thus contribute to healthcare worldwide.
Teaching and Scholarship
![]()
I am a strong proponent of research led teaching, using examples of research in my field to engage students and highlight the need for a thorough knowledge base and analytical ways of thinking.
![]()
I strive to enable students to understand the molecular mechanisms underpinning eukaryotic cell biology – throughout my lectures I discuss experimental evidence that underpins our current understanding of these processes. These lectures are, where possible, supported by lab sessions which give students hands on experience of generating, and analysing data – an important skill for any cell biologist.
![]()
I encourage students to steer tutorials to their interests (within reason!) and find that these small group sessions can lead to lively discussions. Tutorial sessions also give students the opportunity to hone their presentation skills, critically evaluate published literature and obtain feedback on written work.
![]()
Project students in my lab become part of my active research team, usually being closely aligned with work of a PhD student of postdoctoral researcher (although the student always has their own independent projects). Students will have the opportunity to learn a variety of techniques commonly used in cell biology.



