Professor David K. Smith
01904 324181
Email: david.smith@york.ac.uk
Supramolecular soft materials
We are using interactions between molecules to assemble the nanoworld. Systems with dimensions between one and 100 nm in size seem impossibly tiny to the average person – 1000 times smaller than the width of a hair. However, for chemists, we need to bring together multiple molecules to make objects on this scale. This is often referred to as ‘supramolecular’ chemistry – ‘chemistry beyond the molecule.’

Self-assembling nanofibre based on low-molecular-weight building blocks.
We are particularly interested in assembling supramolecular materials that can be physically observed and manipulated and have a wide range of diverse applications. By tuning the molecules we use, and understanding the way they assemble, we aim to develop smart solutions to a range of real-world problems.
Supramolecular gels
Gels are fascinating colloidal materials which surround us in everyday life - from hair gel to jelly babies. Most of the gels we regularly use are made of polymers. These large molecules form a 'solid-like' network within a 'liquid-like' solvent - this mix of solid/liquid properties gives rise to the gel-like behaviour. In our research, we are interested in gels which form from small molecules, rather than polymers. The only way this can occur is through self-assembly, in which the molecules interact with one another to form nanofibres held together by non-covalent interactions like hydrogen bonding.

Supramolecular gel in an upturned sample vial.
The key advantage of these 'supramolecular gels' is that they are highly tunable – using organic synthesis we can create molecules with tailored functional groups, which then become embedded within the gel once assembly has taken place. The non-covalent interactions allow us to translate information from the molecular scale, up to the nanoscale, and then into the actual materials properties of the gel.
We carry out fundamental studies of gel assembly, particularly using mixtures of different building blocks. The non-covalent interactions which underpin such gels can allow order to emerge from complexity. Furthermore, the reversible nature of supramolecular gel assembly means they are highly dynamic and can evolve both over time, and in response to different stimuli.
Industrially-relevant gels with real-world applications
We have developed a new family of hydrogels based on 1,3:2,4-dibenzylidenesorbitol. These molecules are easy to synthesise on a large scale, using low-cost, environmentally-friendly reagents, giving this class of molecules genuine commercial relevance. We are exploring their applications in a variety of fields, including catalysis, sensing, pollution control, drug delivery and tissue engineering.
For example, we have demonstrated that one of our gels can extract precious metals from typical e-waste, creating nanoparticles within the gel network. In this way, we can create gold-loaded gels with applications in soft nanoelectronics, silver-loaded gels with antibacterial activity for use in infection control, and palladium-loaded gels with the ability to catalyse high-value coupling reactions.

Stem cells growing on self-assembled gel.
Some of our gels are compatible with cell growth, and we are currently working with human stem cells in collaboration with Professor Paul Genever (Biology), with the goal of using the self-assembled gel to direct the biological outcomes of the tissue grown within it. The long-term goal is to develop organs for implantation grown from a patient’s own stem cells. This would mean that transplant patients may not need to wait for a matched donor, and would have lower risk of organ rejection. My husband’s own medical experiences, and untimely death, were a key inspiration for our research in this area.
Shaping and patterning supramolecular gels
We are developing innovative methods of shaping and patterning supramolecular gels so that we can achieve both spatial and temporal control over these materials. This moves supramolecular gels beyond being simple soft materials that fill the vial they are made in. Chemical engineering gels in this way allows us to add value and approach a range of distinctive high-tech applications.

Shaped gel created by 3D-printing.
Technologies being used in our laboratory to shape and pattern gels include: moulding, gel bead fabrication, microgel formation, wet-spinning, 3D-printing, photo-patterning and diffusion-based patterning.
Problem-solving with gel technology
Our expertise in gel technology allows us to develop innovative solutions for long-standing practical problems.
For example, organometallic reagents can be very difficult to handle as a result of their high reactivity and low stability. We developed a simple gel, and by formulating organolithium reagents within it demonstrated that instead of requiring handling at low temperatures in inert atmospheres, they could instead be used like many other chemicals and even simply held in a (gloved) hand. We hope to use this approach to transform the shipping, supply, storage and use of such reagents.
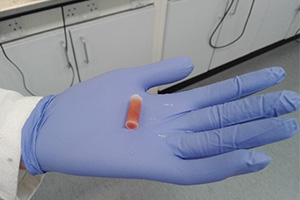
Gel loaded with a highly reactive organolithium being held in a gloved hand in the open lab.
Fascinated by treating brain disorders, one of the biggest problems is enabling drugs to cross the blood-brain barrier. We developed very soft, reversible gels that can be loaded with pharmaceuticals and sprayed into the nasal cavity. The gel-like properties enhanced residence times in the nose and in collaboration with Professor Khuloud Al-Jamal (Pharmacy, King's College London) demonstrated they significantly improved uptake of the drug both into the blood, and directly into the brain.
We regularly work with industrial partners to solve problems, with one example being the development of low-cost gelators with applications in the aviation industry, where they were shown to outperform the current technology.

