Professor Duncan Bruce
+44 (0)1904 324085
Email: duncan.bruce@york.ac.uk
MSc by Research
There are projects available for self-funded MSc(Research) students. Details can be found here and anyone interested in encouraged to make contact.
Liquid crystals and materials chemistry
Career summary
Duncan is a Cumbrian who graduated from the University of Liverpool in 1981 and remained there for his PhD under the supervision of David Cole-Hamilton. His thesis concerned phosphine complexes of PtII and RhI as potential photocatalysts for the decomposition of water. In 1984, he took up a Temporary Lectureship in Inorganic Chemistry at the University of Sheffield and in 1986 was awarded a Royal Society Warren Research Fellowship, which he held there until 1991. He was then appointed Lecturer in Chemistry and was promoted to Senior Lecturer in 1994, in which year he became co-director of the Sheffield Centre for Molecular Materials. In 1995, he took up the Chair in Inorganic Chemistry at the University of Exeter and, following Exeter's disastrous closure of Chemistry in 2005, Duncan assumed his present position as Professor of Materials Chemistry in York and served as Head of Department from 2015-2021.
He was President of the Royal Society of Chemistry Materials Division from 2006-2009, Chair of the British Liquid Crystal Society from 2009 to 2011 and was an elected member of RSC Council (2011 to 2015) and Chair of its Audit Committee (2012 to 2015). He was Chair of the Awards Working Group (2017-21) and was Chair of the Trustees of the Pension Scheme (2019-23) and a Member of the Disciplinary Committee (2016-22).
His work has been recognised by various awards including British Liquid Crystal Society's first Young Scientist prize (1990), the RSC's Peter Day Award (2014), Tilden Prize (2010), Corday-Morgan Medal and Prize (1996) and Sir Edward Frankland Fellowship (1994/95) and the Gold Medal Award of the Chirantan Rasayan Sanstha, India (2022). He has held visiting positions in Argentina, Australia, Chile, France, Japan, Italy and Taiwan.
Research interests
The group has a range of interests, a common factor of most being liquid crystals. Examples of current projects include:
Luminescent Liquid Crystals for OLED Applications
Orthometallated 2-phenylpyridine derivatives of some third-row transition metals have desirable photophysical properties as spin-orbit coupling allows ready access to the triplet manifold and theoretical unit emission efficiency. However, if such complexes can also show liquid crystal properties then additional effects may be realised, for example enhanced carrier mobilities or polarised emission. Our photophysical work in this area is carried out in collaboration with Professor Gareth Williams in Durham, computational work (and synthetic work in the gold chemistry) is in collaboration with Professor Jason Lynam, while OLED devices are prepared in collaboration with Professor Yafei Wang of Changzhou University in China.
Gold(III) Chemistry: C,N,C pincer complexes of gold(III) functionalised with acetylide co-ligands are known to be phosphorescent and so we have prepared the complexes shown below:

Where there are two alkoxy chains on the pincer ligand then emission quantum yields are rather low (ca 2%), but using four give much higher values (ca 35%). All form columnar mesophases (mainly hexagonal), whose stability and range increases with the number of appended alkoxy chains. Devices have been prepared and show external quantum yields of up to ca 7% (J. Mater Chem. C, 2021, 9, 1287-1302).
When fluorinated chains are used, the mesomorphic properties are similar (although the columnar phases tend to be a little more stable) and the solution quantum yields are the same as the computational work carried out shows that the chromophore is associated with the pincer ligand. However, the fluorinated chains suppress solubility of the complexes in OLED host materials, leading to poor device parameters (ACS Omega, 2022, 7, 24903-24917).
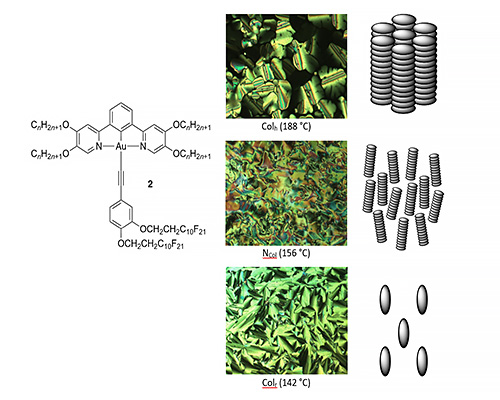
However, for one complex (2), the liquid crystal behaviour found was remarkable so that on cooling from the isotropic liquid, a Colh phase was observed followed by a nematic phase and then a Colr phase (see below). Thus, a very disordered (nematic) phase is found between two much more ordered columnar phases – an observation made once previously in the 1980s in a series of related truxenes. We believe that the behaviour is driven by the amphiphilic nature of the complex on account of the mutual immiscibility of the hydrocarbon and fluorocarbon segments (Liq. Cryst., 2022, 49, 1162-1173).
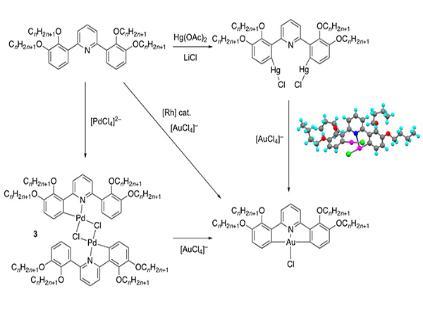
Gold(III) complexes of this type are prepared from the ligands via intermediate mercury(II) complexes as direct metalation by gold is as best difficult but, evidently, the use of mercury makes these materials unattractive in many ways. We have recently shown (Dalton Trans., 2023, 52, 872-876) that the gold complexes may be obtained through intermediate palladium(II) complexes (3) as well as via a Rh-catalysed route reported by Nevado and co-workers for 2-phenylpyridines. As part of this study, we found that some C,N,C pincers form stable and isolable complexes, an example of which is shown below.
Platinum Chemistry: The terdentate complexes (3) of platinum(II) form columnar phases but the nature of the molecular organisation therein depends on how the mesophase is accessed. Thus, slow cooling leads to the formation of a monomer structure with yellow/orange emission, while fast cooling leads to organisation with short Pt…Pt interactions as evidenced by red emission from a MMLCT state. The two emissive states can be interconverted using a combination of temperature and mechanical disturbance (ACIE, 2008, 47, 6286-6289).
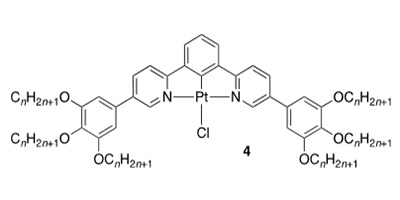
Complexes of platinum(II) based on extended 2-phenylpyridines (5 – with or without fused C5 ring) show nematic and smectic A phases and, depending on the nature of R/R', show emission quantum yields ranging from zero (R = R' = CF3) to 0.7 (R = CH3, R' = CF3). Quenching of the emission where R = R' = CF3 is attributed to the LUMO now being situated on the ß-diketonato ligand rather than the 2-phenylpyridine, which was shown both by calculation and by the EPR spectrum of the reduced complex (Chem. Mater., 2009, 21, 3871-3882, Dalton Trans., 2012, 41, 14244-14256). Interestingly, attempted preparation of 2 by reacting the ligand with cis-[PtCl2(S-dmso)2] in AcOH leads to the non-emissive, liquid-crystalline dimer of platinum(III) 6, with oxidation mediated by dmso/H+ (J. Am. Chem. Soc., 2010, 132, 10689-10691).
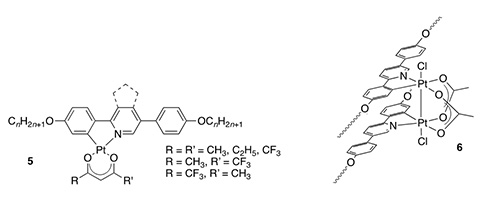
We have also reported emissive, liquid-crystalline derivatives of platinum(IV) (7), which show a ligand-based emission. The paper also reports a direct reaction from platinum(II) and 2-phenylpyridines to realise platinum(IV) products (Chem. Eur. J., 2018, 24, 19010-19023).
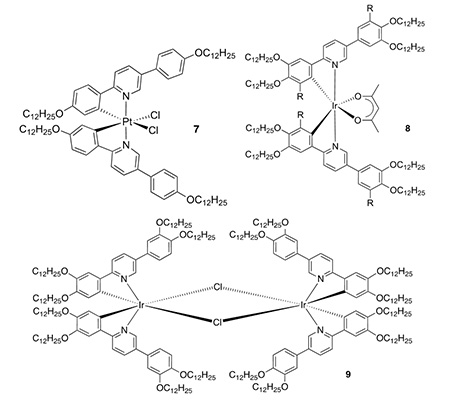
Iridium(III): Also with octahedral complexes, we reported on liquid-crystalline emitters based on iridium(III) (8 – J. Am. Chem. Soc., 2011, 133, 5248-5251) and with dimeric derivatives (9), we recorded some good emission quantum yields (Angew. Chem. Int. Ed., 2012, 51, 95-98).
As part of our active collaboration with Professor Yafei Wang at Changzhou University in China, we have published with his group on liquid-crystalline OLED materials based on platinum(II) and iridium(III), and others concerned with aggregation-induced emission, circularly polarised emission and through-space TADF.
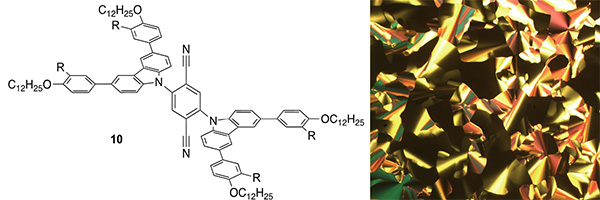
Liquid-crystalline TADF Materials: As well as functionalising phosphorescent metal complexes to confer liquid crystal properties, we have applied the same thinking to TADF chromophores where 10 has a TADF PLQY of 18% (R = H) and 5% (R = OC12H25). These are respectively lower in related materials where there are four carbazoles attached to the terephthalonitrile core. Liquid crystal properties result only where the carbazoles bear four dodecyloxy chains (J. Mater. Chem. C, 2021, 10, 6528-6535).
Changing the substitution pattern (11) lower the liquid crystal transition temperatures and gives superior device performance compared to the more symmetric materials above (Phys. Chem. Chem. Phys., 2022, 24, 22115-22121).
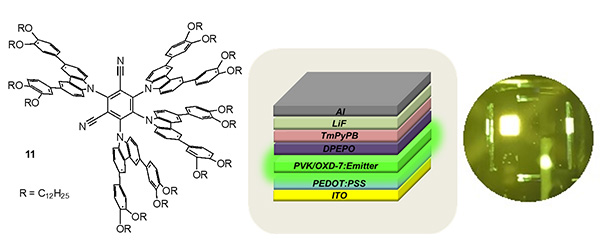
Examples (12) have also been prepared and characterised as part of our collaboration with Wang (ACS Appl. Mater. Interfac., 2022, 14, 15437–15447).
Ionic Liquids and Liquid Crystals
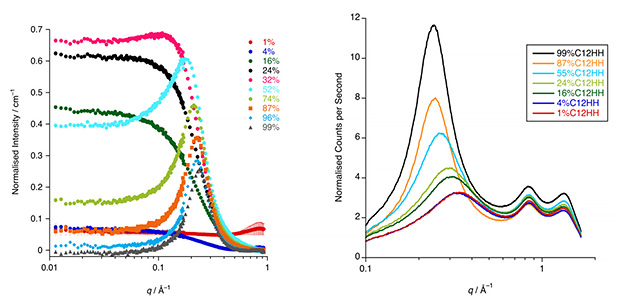
1. The Surface and Bulk Structure of Ionic Liquids. This is a collaboration with John Slattery in York, Ken McKendrick and Matt Costen of Heriot Watt University, Tim Minton at Montana State University and Peter Wasserscheid and Marco Haumann in Erlangen. The project is concerned with the dynamic scattering of gases from the surface of the ionic liquids in order to probe their composition with O(3P) atoms are used as reactive probes of the surface structure and dynamics. The experiments at Heriot Watt and Montana utilise very different O(3P) atom energies, allowing for different chemical groupings to be sampled. Studies showed (J. Phys. Chem. B. 2017, 121, 6002-6020) that in mixtures of short- and long-chain ionic liquids based on the methylimidazolium cation, long-chain homologues concentrate preferentially at the surface, while complementary small-angle X-ray (SAXS) and neutron scattering (SANS) studies of the bulk showed local nanosegregation of the long-chained species (Farad. Discuss., 2018, 206, 265-289).
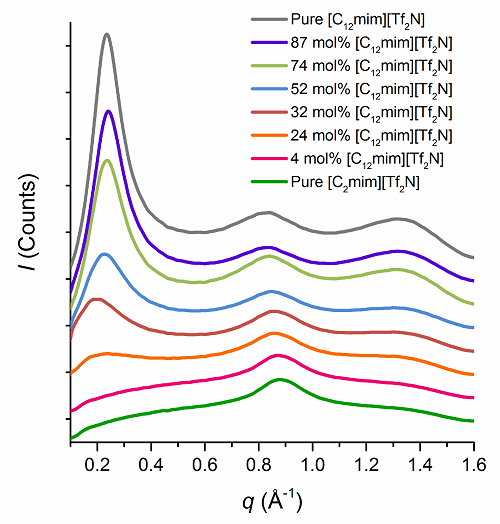
SAXS data. Reproduced by kind permission of the ACS.
The bulk structure of the ionic liquid mixtures has been probed by a combination of SANS and SAXS complemented by MD simulations carried out in collaboration with Karina Shimuzu and Ze Nuno Canongia Lopes from Lisbon (Phys. Chem. Chem. Phys., 2022, 24, 15811–15823).
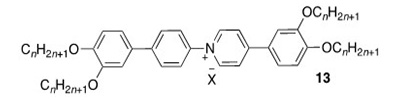
2. N-Phenylpyridinium Liquid Crystals. We reported the synthesis and phase behaviour of tetracatenar ionic mesogens based on the N-phenylpyridinium cation (13) where we have observed an unprecedented stabilisation of the columnar phase and the rare observation of a SmA phase (Molecules, 2021, 26, 2653). The mesomorphism of silver(I) complexes of the 3,4-dialkoxyphenylpyridines has also been reported (J. Mol. Liq., 2022, 362, 119707).
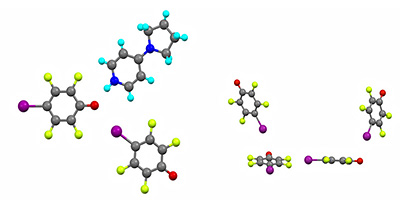
3. Triazolium Liquid Crystals. With John Slattery, and Ivana Pibiri and Andrea Pace (Palermo, Italy) we have prepared examples of triphilic (hydrocarbon, fluorocarbon and ionic) mesogens based on a 1,2,4-triazolium cation (14) which show the SmA phases typical of ionic materials (J. Mol. Liq., 2021, 321, 114758). Triphilic organisation is seen in the isotropic state and mesophase, and is evidenced in the solid state in the single crystal structure (below) and in an extensive crystallographic study that has been published (CrystEngComm, 2022, 24, 7852-7860).
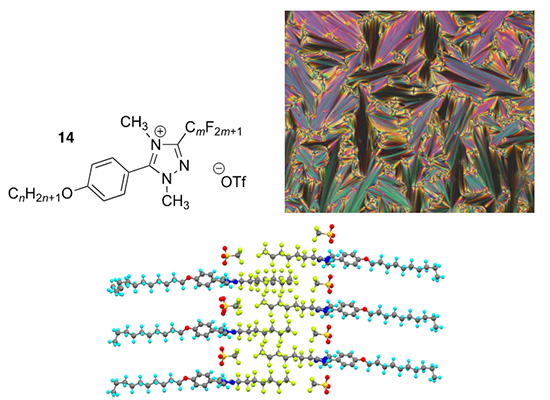
Reproduced by kind permission of the Royal Society of Chemistry.
Halogen-bonded Liquid Crystals and Co-crystals
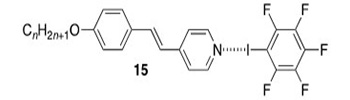
Following our report of the first example of a halogen-bonded liquid crystal (15) back in 2004 (J. Am. Chem. Soc., 2004, 126, 16-17), we worked extensively in this area (see eg Chem. Eur. J., 2010, 16, 9511-9524) and have recently published a critical review of the area (Helv. Chim. Acta., 2023, 106, e202300008).
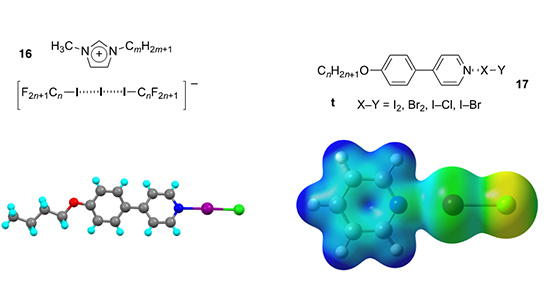
In collaboration with Resnati and Metrangolo, we reported on the liquid crystal properties of ionic halogen-bonded materials (16) where the liquid crystallinity was driven by the halogen-bonded anion (Angew. Chem. Int. Ed., 2016, 55, 6300-6304). Most recently, we have reported on the structure and mesomorphism of some halogen-bonded complexes of interhalogens (17), which tend to show SmA phases (CrystEngComm, 2023, 25, 1683-1692). Theoretical aspects of these studies have been carried out in collaboration with my colleague Peter Karadakov (see also. J. Phys. Chem A, 2012, 116, 10621 and Cryst. Growth Des., 2010, 10, 3710).
We also branched out a little into aspects of crystal engineering (see e.g. Chem. Eur. J., 2014, 20, 6721-32, CrystEngComm, 2014, 16, 4254-4264).