Dr Matthew Jones
Research interests
I combine a background in marine chemistry, environmental science and analytical chemistry with ideas from chemical engineering and bio/microbial chemistry while working in an atmospheric chemistry laboratory. It goes to show just how well-connected we all are.
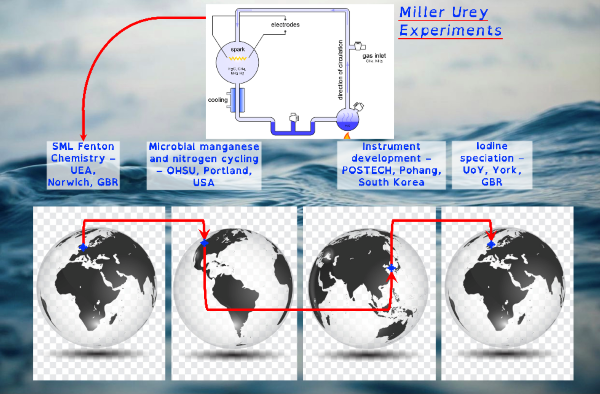
I do this to understand the organo-geo-redox-chemistry of reactive intermediate species during advanced oxidation processes controlled by environmental processes. A minefield of terms but the most succinct way of stating my research and why I have chosen a not-so-direct career path. This fundamental research is essential because these systems control the biogeochemical cycling of the microbially essential trace nutrients iron and manganese. They control how microbially derived organic carbon interacts with inorganic chemicals. They control the movement and cycling of toxic metals. These advanced oxidation processes provide feedback mechanisms that control atmospheric phenomena such as cloud formation and ozone toxicity. Though organo-geo-redox-chemistry of reactive intermediate species is ubiquitous, from soils to the deep ocean, where these environmental cycles are most likely to occur is in the light-exposed layer of the global ocean.
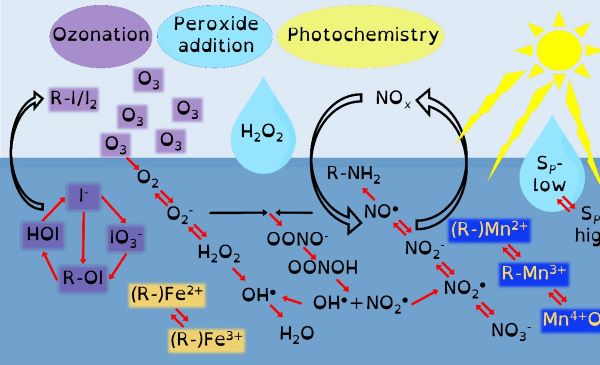
Through chemical engineering advanced oxidation processes, which are enviromimetics of photochemistry (UV-irradiation), ozone deposition at aqueous surfaces (ozonolysis) and mixing of seawater and freshwater (changes in ionic strength), we can control organo-geo-redox-chemistry. Knowledge of biochemistry is important because extracellular microbial processes use these very same reactive intermediate chemicals in warfare, as nutrients and to make their life mildly less inhospitable. Advanced oxidation processes should force chemicals to dance with one another in repeatable reaction motifs, which microbial processes will then draw into another pattern. The environment is balanced between advanced oxidation processes and biochemistry yet we possess only basic knowledge of environmental advanced oxidation processes, compared to that in biochemistry.
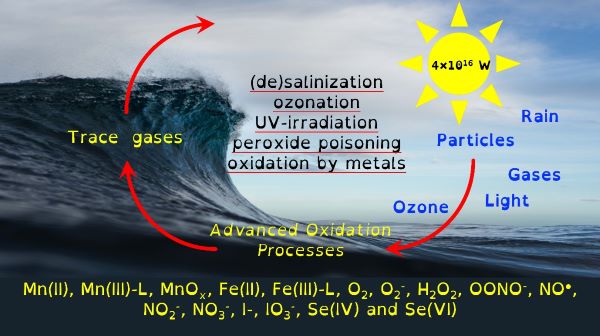
Our environment is swamped by naturally occurring energetic chemicals that have strong oxidising or reducing properties but are relatively short-lived. A key aspect of their short but happy lives is that when they are excited they like to dance and while dancing will choose many different partners to spread their energy to. These short-lived happy chemicals are typically intermediate chemicals during the transformation from one stable chemical into another. The most well-known of these transformations is that of oxygen into water. Spontaneity is slow, so there are initiating processes that provide the initial energy that begins a naturally choreographed dance of chemicals.
Selected publications
- Jones, M.R., Chance, R., Dadic, R., Hannula, H.-R., May, R., Ward, M., Carpenter, L.J., 2022. Environmental iodine speciation quantification in seawater and snow using ion exchange chromatography and UV spectrophotometric detection. Analytica Chimica Acta tbd, tbd.
- Jones, M.R., Lee, K., 2020. Precipitation of hydrogen peroxide during winter storms and summer typhoons. Science of The Total Environment 733, 139377. https://doi.org/10.1016/j.scitotenv.2020.139377
- Jones, M.R., Luther, G.W., Tebo, B.M., 2020. Distribution and concentration of soluble manganese(II), soluble reactive Mn(III)-L, and particulate MnO2 in the Northwest Atlantic Ocean. Marine Chemistry 226, 103858. https://doi.org/10.1016/j.marchem.2020.103858
- Jones, M.R., Tebo, B.M., 2021. Novel manganese cycling at very low ionic strengths in the Columbia River Estuary. Water Research 207, 117801. https://doi.org/10.1016/j.watres.2021.117801
